Researchers at University College London (UCL) and UCL Hospital (UCLH) have achieved a significant milestone by completing the world’s first trial of a groundbreaking therapy designed to restore hearing loss. The REGAIN trial, which was featured in a publication by Nature Communications, marked a pioneering endeavor to investigate a treatment focused on a gamma-secretase inhibitor called LY3056480, aiming to rejuvenate lost hearing capabilities. While the therapy did not universally restore hearing among adults with mild to moderate hearing impairments, a detailed analysis of the trial results demonstrated observable changes in hearing tests for select patients, suggesting a promising level of activity of the drug within the inner ear.
These encouraging efficacy signals emphasize the necessity for further advancement of LY3056480, leveraging the insights gained from this pivotal trial.
The participants in the trial, ranging from 18 to 80 years old and originating from the United Kingdom, Germany, and Greece, presented with mild to moderate degrees of hearing loss. The phase 1 trial involved 15 patients, establishing the safety and tolerability of the treatment, while the subsequent phase 2a trial, comprising 44 patients, aimed to evaluate the drug’s effectiveness.
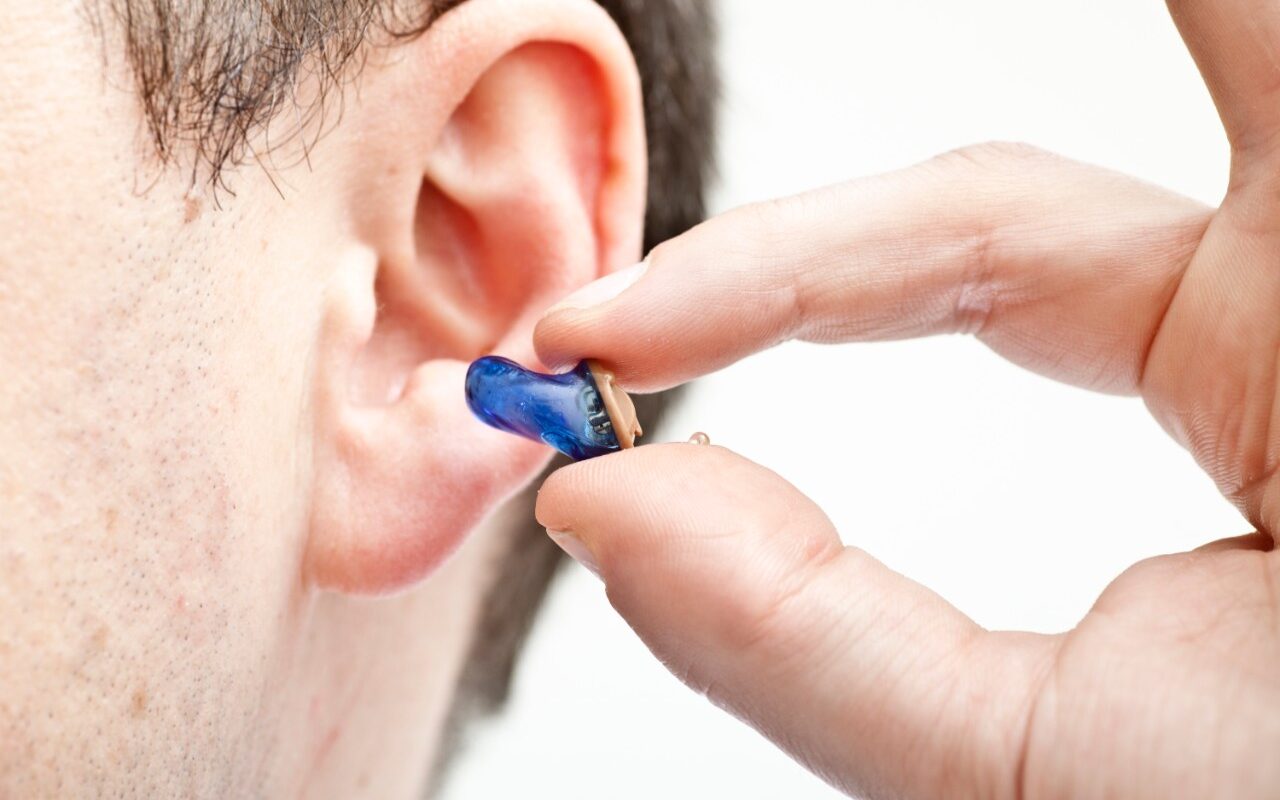
During the trial, patients received three injections of the drug administered through the eardrum, followed by pre- and post-treatment assessments of their auditory function through various tests.
One test focused on determining the quietest detectable sounds by the participants, while another assessed their ability to decipher word sounds amid background noise, a common challenge faced by individuals with hearing loss.
At the 6 and 12-week intervals post-treatment commencement, 45% of participants reported identifying sounds at least 10 decibels less than their initial threshold. However, the trial’s benchmark for success, an average improvement of 10 decibels or more across three sound frequencies, was not uniformly met by the observed hearing changes.
Hearing loss stands as a prevalent sensory impairment globally, representing a significant area with pressing unmet needs. The leading cause of hearing loss is often attributed to the gradual degeneration of inner ear sensory hair cells or their functionality. Presently, the standard treatment for hearing loss remains the usage of hearing aids, which aid communication but do not address the root cause or fully restore natural hearing.
Given the intrinsic incapacity of inner ear sensory hair cells to regenerate naturally, age-related hearing loss persists as a prevalent concern.
Although the long-standing belief held that the damage to inner ear hair cells was irreversible, research in animal models hints at the potential regeneration of functional inner ear sensory hair cells through the administration of a gamma-secretase inhibitor.
Led by Professor Anne GM Schilder from UCL Ear Institute and UCLH Royal National ENT, Eastman Dental Hospitals, and NIHR UCLH Biomedical Research Centre, the REGAIN trial (Regeneration of inner ear hair cells with gamma-secretase inhibitors) represented a groundbreaking exploration into regenerative hearing drug therapies. This international initiative was spearheaded by an EU Consortium and featured clinical collaborators in Germany and Greece, alongside Audion Therapeutics.
Reflecting on the outcomes of the trial, Professor Schilder recognized the invaluable insights garnered and emphasized the critical role these findings will play in shaping future investigations into similar regenerative hearing treatments. Enhancing patient selection criteria, comprehending the underlying mechanisms of inner ear hearing loss, and developing more precise diagnostic tools were highlighted as crucial areas for advancement.
To further this groundbreaking work, the UCLH Biomedical Research Centre team, in collaboration with other BRCs, has established the NIHR Hearing Health Informatics Collaboration (HIC) to amalgamate anonymized hearing data from NHS hospitals across the UK for advanced computational analysis.
Moreover, the introduction of a patient registry known as HEDGE enables individuals with hearing loss to engage in research activities aimed at unraveling the genetic, environmental, and molecular factors contributing to hearing impairments.
The overwhelming patient interest in participating in the REGAIN trial underscored the substantial unmet clinical needs within the realm of hearing loss.
As Professor Schilder affirmed, the resonance from over 5,000 individuals with hearing loss expressing a desire to engage in research epitomizes the global demand and urgency for innovative clinical solutions.
The pioneering research conducted at the UCL Ear Institute, elucidating the role of specific proteins in inner ear hair cells, has paved the way for transformative hearing therapies, exemplified by the regenerative approach trialed in REGAIN.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it