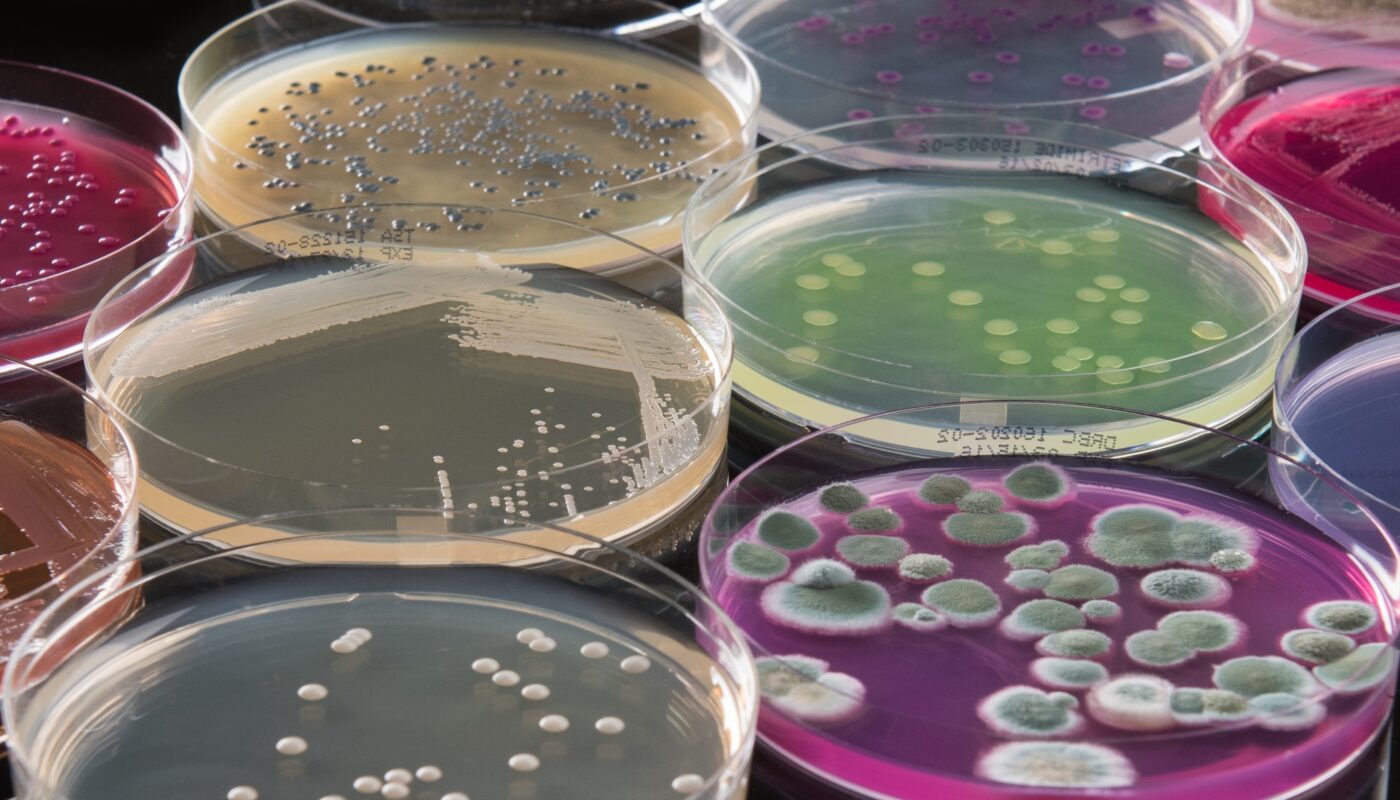
The pharmaceutical industry is continuously evolving to meet the growing healthcare demands of the world. One area that is seeing major growth and innovation is the development of microbial-derived active pharmaceutical ingredients (APIs). These APIs produced through microorganisms like bacteria and fungi offer several advantages over traditional chemical synthesis and are poised to disrupt the global API market.
The Advantages of Microbial APIs
Microbial fermentation allows for a more sustainable and environmentally friendly production process when compared to chemical synthesis. Through fermentation, microbes metabolize sugar, gases, or other organic compounds and secrete the desired API. This avoids the use of harsh and toxic chemicals that are commonly used in chemical synthesis. Additionally, fermentation typically yields higher selectivity and purity compared to multi-step chemical synthesis which require extensive purification.
From an economic standpoint, microbial processes offer lower production costs over the long run. Once an optimized strain is developed, fermentation yields can be increased through scale-up in large bioreactors without significantly adding to production costs. The fixed costs are also lower compared to building and operating chemical plants. This makes microbial APIs a more viable option for producing high-volume drugs in a cost-effective manner.
The Rise of Generic Drug Manufacturing
The expiration of patents and regulatory exclusivity periods for many blockbuster drugs has lead to a surge in generic drug manufacturing globally. According to a report by Grand View Research, the global generics drug market size was valued at $338 billion in 2020 and is expected to reach over $500 billion by 2028. A major driver of this growth is the production of low-cost generic versions of drugs going off-patent by pharmaceutical companies in developing markets like India, China, and Brazil.
Microbial Fermentation offers a scalable and cost-effective manufacturing solution for producing the APIs required for many such generic drugs. Well-established fermentation processes allow generic drug makers to consistently supply quality generic medicines at a fraction of the cost of originator brands. Some of the top-selling generic drugs developed through fermentation include antibiotics like penicillin, cephalosporins, and anti-fungal drugs. The low-cost generic drug boom has spurred API fermentation capacity expansion across Asia and Latin America.
Advances in Synthetic Biology and Metabolic Engineering
While traditional fermentation processes have enabled large-scale production of common microbial APIs for decades, advances in synthetic biology and metabolic engineering are opening up new opportunities. Engineers can now reprogram microbes to act as cell factories for producing high-value APIs that are difficult or uneconomical to make through chemical or traditional fermentation routes.
By modifying metabolic pathways and introducing novel enzymes and biosynthetic gene clusters, microbial cell lines have been developed that churn out precursor molecules, semi-synthetic APIs, cannabinoids, flavonoids and other natural products at industrial scales. For example, engineered strains of E. coli and yeast now produce artemisinin, opioids, cannabinoids and flavonoids which were previously extracted from plants in limited quantities.
This has paved the way for a new class of biologic drugs and nutraceuticals derived entirely or partially through fermentation instead of agricultural extraction or chemical synthesis. As the tools of synthetic biology evolve, the library of ‘microbial chemical factories’ will continue to expand meeting more of the API needs of the pharmaceutical and supplements industries.
Challenges and Regulations
While microbial fermentation offers compelling advantages, there are also regulatory and technological challenges to overcome for its wider adoption in API manufacturing. Stringent regulatory requirements exist around characterizing biologically produced APIs and ensuring consistent quality, purity, and safety. Furthermore, the complexity of fermentation processes requires sophisticated control and monitoring which adds to production costs compared to simpler chemical syntheses.
Regulators also want assurances that no toxins or undesirable metabolic byproducts are generated during scale-up. Any genetic modifications made to microbes must be rigorously evaluated for their safety. As with any biological system, inherent variability also exists between fermentation batches that needs to be tightly controlled. Developing robust downstream processing capabilities to efficiently extract, purify and formulate microbial APIs remains an active area of research.
The stringent manufacturing practices (GMP) regulations that fermentation facilities must adhere to also impacts the setup and operating costs. However, as production scales increase and processing technologies advance, these regulatory requirements and associated costs are expected to become more manageable. Overall, harmonization of global regulatory guidelines will be critical for the microbial API industry to achieve its full potential as a global enterprise.
With these advantages and challenges, microbial fermentation is sure to play a growing role in meeting the world’s burgeoning need for low-cost, sustainably produced APIs. Analysts project the global microbial API market to be worth over $60 billion by 2030. Technologies like continuous production bioreactors, in-situ product recovery, and advanced process analytics are being developed and adopted to make fermentation ever more efficient, cost-effective and compliant with stringent regulations.
As synthetic biology capabilities progress, the toolbox available for metabolic engineering will expand exponentially. Collaborations between industry and academia will unlock new pipelines for producing novel APIs, flavors, fragrances and specialized intermediates in microbes. With targeted policy support and global partnerships focused on R&D, the microbial API industry is well positioned to deliver innovative, affordable medicines to a greater portion of humanity and drive a truly sustainable bioeconomy. The future looks bright for this exciting emerging sector.