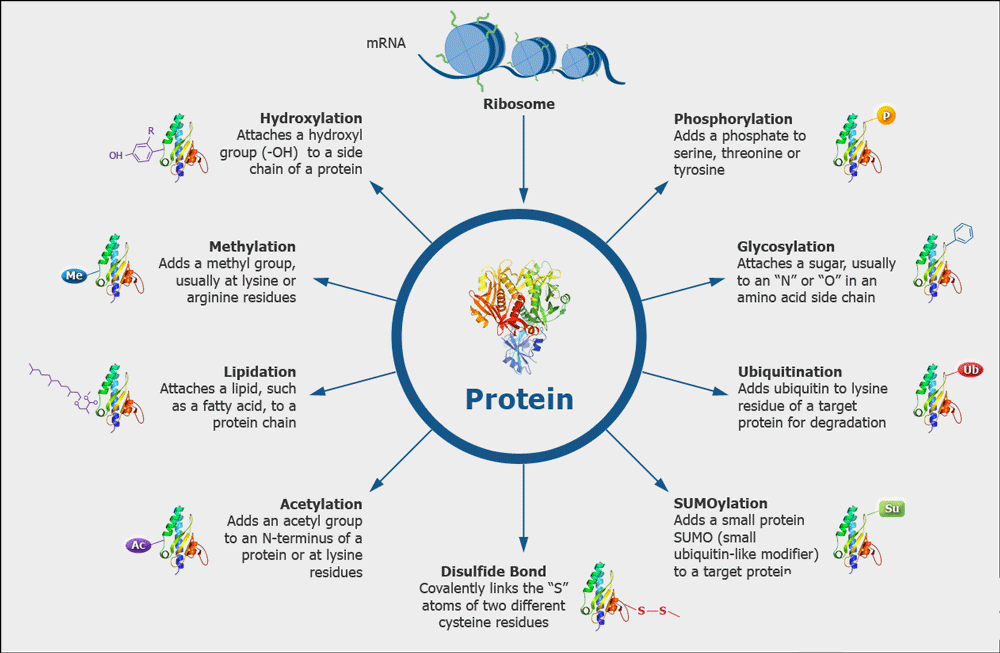
Scientists have made a breakthrough in understanding how post-translational modifications (PTMs) of proteins can impact the development and progress of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. These diseases are characterized by the accumulation of misfolded protein aggregates in the brain, known as amyloid fibrils, which lead to cell dysfunction and death.
The research, led by Hilal Lashuel at EPFL and Matthew R. Pratt at USC, focused on the protein alpha-synuclein, which is associated with the formation of amyloid fibrils in Parkinson’s disease. Specifically, the scientists studied a particular modification called O-linked β-N-acetylglucosamine (O-GlcNAc), which adds a sugar molecule to specific serine or threonine residues in the protein. This modification has been linked to various biological processes, including protein aggregation and neurodegeneration.
Previous studies by Pratt and Lashuel’s teams suggested that increasing O-GlcNAc modification could have therapeutic potential in the early stages of neurodegenerative disease. It was found that this modification could alter the properties of protein aggregates, slowing down amyloid aggregation and potentially protecting neurons.
To further investigate, the researchers used innovative chemical methods to produce modified alpha-synuclein fibrils, with the assistance of the University of Pennsylvania and UT Southwestern Medical Center. They also utilized cell and animal models to study how O-GlcNAc affects the pathogenic properties of alpha-synuclein. Cryo-electron microscopy was employed to observe the modified fibrils.
The study revealed that increased O-GlcNAc modification resulted in fibrils with distinct structural and biochemical features. These fibrils demonstrated a significantly reduced ability to seed aggregation in neurons and animal models of Parkinson’s disease. Interestingly, these modified fibrils could seed aggregation in vitro but not in living organisms.
The findings highlight the importance of the cellular environment in determining the pathogenicity of alpha-synuclein. “Our findings show that this environment in the cell plays an important role in determining the pathogenicity of this protein,” said co-first author Anne-Laure Mahul.
The study suggests that modifications like O-GlcNAc could potentially modulate the pathogenicity of alpha-synuclein, opening up new avenues for research and potential treatments. For example, targeting the O-GlcNAc modification process could lead to therapies that alter the progression of Parkinson’s by influencing the ability of pathogenic alpha-synuclein species to spread to different regions of the brain.
This research provides valuable insights into the molecular determinants of amyloid fibril pathobiology and offers new therapeutic targets for preventing amyloid growth and spreading during various stages of disease development and progression.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it