Introduction
The patent foramen ovale (PFO) is a flap-like opening between the left and right atria of the heart that normally closes after birth. In about 25% of the population, this opening fails to close fully or reopens, resulting in a PFO. While a PFO is considered a normal anatomical variant in most people, it has been associated with several medical conditions like migraine headaches, strokes and decompression sickness. In recent years, PFO closure devices have emerged as an effective treatment option for selected patients with PFO-related conditions. This article provides an overview of PFO closure devices and their role in management of patients with a PFO.
Types of PFO Closure Devices
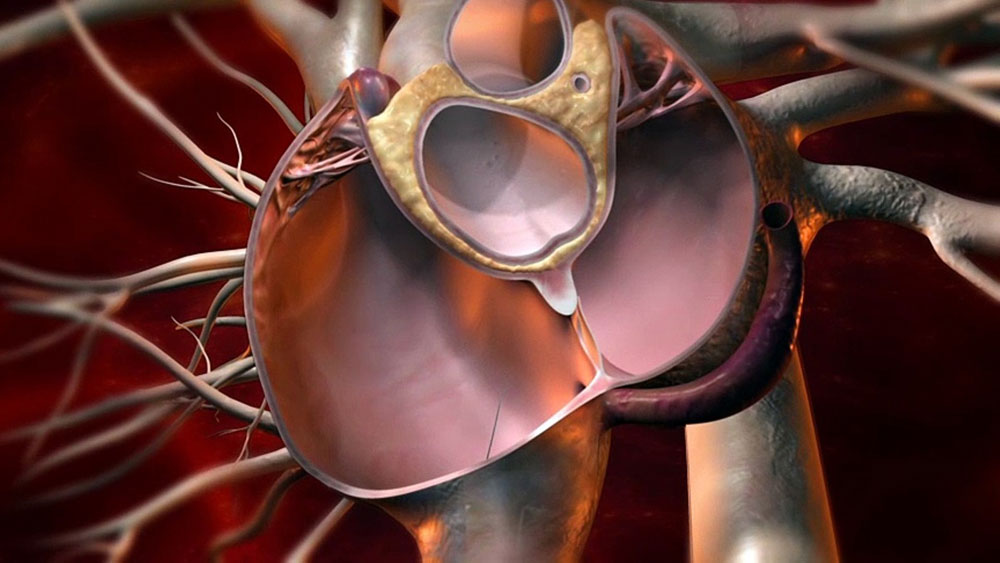
There are different types of self-expanding nitinol mesh devices that are used to close a patent foramen ovale percutaneously without the need for open heart surgery. Some of the common FDA approved PFO closure devices include:
– Amplatzer PFO Occluder: Manufactured by St. Jude Medical, it is a self-expanding double disk device made of nitinol mesh used to close PFOs up to 18mm in diameter.
– Gore Cardioform Septal Occluder: Developed by Gore Medical, it is a self-expanding umbrella-shaped device deployed via a delivery cable. It consists of a left atrial disc and a right atrial disc connected by a central waist.
– Helex Septal Occluder: Manufactured by W.L. Gore Associates, it is a self-centering nitinol mesh device with a left atrial disc and a right atrial disc connected by a short central waist.
– Cera PFO Occluder: A self-expanding nitinol mesh device from Lifetech Scientific made of braided nitinol wires forming a left and right atrial disk. It is pre-loaded in a user-friendly catheter based delivery system.
Mechanism of PFO Closure
All PFO closure devices are delivered percutaneously through the femoral or jugular vein under fluoroscopic guidance. The device is folded and loaded inside a delivery catheter. Using trans-esophageal echocardiography (TEE) and fluoroscopy, the tip of the delivery catheter along with the compressed implant is guided through the PFO into the left atrium.
Once in position, the implant is slowly deployed. The left atrial disk self-expands and anchors securely to the left atrial septum. The central waist expands in the PFO tunnel. Then the right atrial disc self-expands and anchors into the right atrial septum. This helps appose the septum and close the PFO permanently.
Indications for PFO Closure
Patent Foramen Ovale (PFO) Closure Devices closure is usually recommended for patients who have had a cryptogenic (cause unknown) stroke or transient ischemic attack (TIA) and are found to have a PFO on testing. It is also considered for patients experiencing recurrent migraines with aura if their migraines are attributed to the PFO. Other indications include decompression sickness or retinal vein occlusion in divers or air travelers with a PFO. Patients with established venous thromboembolism or pulmonary embolism may also be candidates if the PFO is considered the cause.
PFO Closure Process and Outcomes
The procedure is usually conducted under conscious sedation in a catheterization lab. Patients are given anticoagulant/antiplatelet medication before and after the procedure based on individual risk factors. During the procedure, TEE is used to guide closure device placement and ensure closure immediately after placement. Most patients can resume normal activity within 1-2 days.
Long term studies have shown PFO closure to be a safe and effective method for secondary stroke prevention in select patients. Up to 95% PFO closure is achieved post-procedure. Studies comparing device closure to medical management have shown 65-70% relative risk reduction for recurrent strokes at 2 years. There is also evidence of significant migraine improvement in over 60% patients up to 1 year after closure. However, long term data beyond 5 years is still awaited. Complication rates are low at 1-2% and include device embolization or residual shunt.
Conclusion
In summary, percutaneous PFO closure with self-expanding devices has emerged as a minimally invasive treatment alternative to medical management or open heart surgery for selected patients with PFO-related conditions like recurrent strokes, migraines and decompression illness. Long term studies continue to evaluate safety, efficacy and cost-effectiveness of PFO closure against medical therapy alone. With advances in technology, various newer smaller and easier to deploy devices are being introduced which may expand the eligibility of patients who can benefit from this intervention.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it.