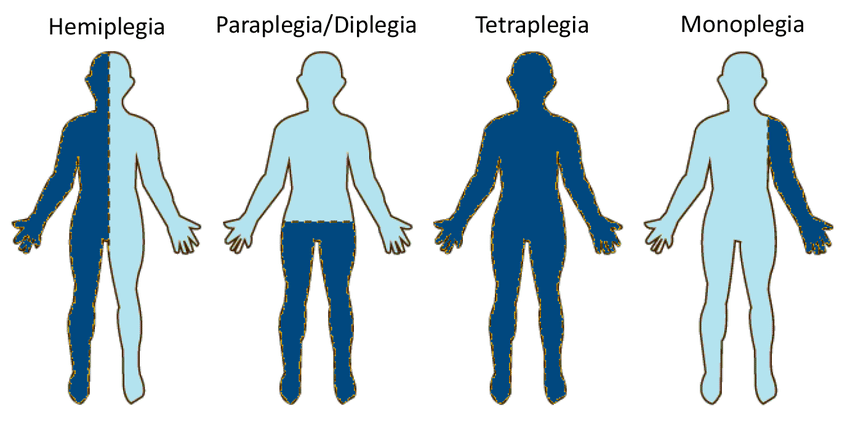
Spastic paraplegia refers to a rare, genetic neurological disorder that results in progressive stiffening of the leg muscles. The primary symptoms include progressive weakness and stiffness in the lower limbs along with hyperreflexia. Gene therapy has emerged as a promising treatment modality for various inherited neurological disorders by correcting gene mutations responsible for causing diseases. Advancements in gene therapy approaches, such as viral vectors and genome editing tools, are expected to aid in developing effective treatments for spastic paraplegia in the coming years.
The global spastic paraplegia 50 market is estimated to be valued at US$ 130 million in 2024 and is expected to exhibit a CAGR of 10% over the forecast period of 2024 to 2031.
Key Takeaways
Key players: Key players operating in the global spastic paraplegia market are Pfizer, Sanofi, Novartis, GlaxoSmithKline, Johnson & Johnson, Merck, AstraZeneca, Bayer, Boehringer Ingelheim, Amgen, Biogen, Takeda Pharmaceutical, AbbVie, Bristol-Myers Squibb, Astellas Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, and Novo Nordisk.
Key players in the spastic paraplegia market are focusing on collaborations to advance gene therapy research. For instance, in 2022, Xenon Pharmaceuticals collaborated with Neurocrine Biosciences to develop gene therapies for various neurological diseases including spastic paraplegia.
Key opportunities: High unmet medical needs in the Global Spastic Paraplegia 50 Market Trends paraplegia present significant growth opportunities for players. Furthermore, increased funding for rare disease research is also boosting opportunities.
Technological advancements: Advancements in gene therapy tools such as AAV vectors and base editing are expected to aid in developing effective treatments. CRISPR/Cas9 gene editing is also a promising approach being explored for treating hereditary spastic paraplegia.
Market Drivers
Increasing research funding: Raised awareness about rare genetic neurological disorders along with increased funding from government and private organizations is driving research on novel treatments for spastic paraplegia. For instance, the National Organization for Rare Disorders awarded grants worth US$ 25 million in 2021 for various rare disease research programs including those focused on spastic paraplegia. This will accelerate drug development efforts.
Current Challenges in the Global Spastic Paraplegia Market
The global spastic paraplegia market faces several challenges currently. Due to low disease awareness, many cases remain undiagnosed. This acts as a major hindrance for the market. Further, the complex nature and rarity of the condition makes treatment difficult. Currently, there is no cure available and existing treatment options only provide symptomatic relief. Development of novel therapies requires extensive research which is expensive. This deters many pharma players from investing in this market. High cost of customized rehabilitation also restricts growth. However, ongoing research and diagnosis improvements are expected to address some of these challenges in the coming years.
SWOT Analysis
Strength: Growing research focus on genetics and pathophysiology of different types provides insights for therapy development.
Weakness: Low disease prevalence makes commercialization of therapies difficult.
Opportunity: Developing early diagnosis techniques through biomarkers can improve treatment outcomes.
Threats: High attrition rate in drug development due to disease complexity and heterogeneity poses risk.
Geographically, the North American region currently holds the major share of the global spastic paraplegia market, both in terms of value and volume. This is mainly attributed to rising healthcare spending, presence of leading market players, and growing research activities in the region. The Asia Pacific region is expected to witness the fastest growth over the forecast period due to improving access to healthcare facilities, increasing awareness about rare diseases, and rising disposable incomes in emerging countries.
The European market also captures a notable value share due to supportive government initiatives for orphan drug development and availability of advance healthcare infrastructure across major countries. On the other hand, the Latin American and Middle East & African regions are projected to provide lucrative opportunities for market expansion in the coming years. This will be driven by growing medical tourism, private sector investments, and expanding diagnosis capabilities.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it.