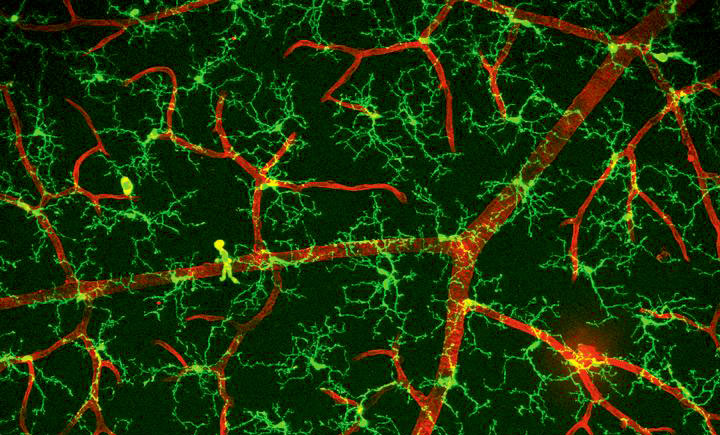
Microglia are a specialized group of immune cells within the brain that typically play a protective role. However, in the case of neurodegenerative conditions such as Alzheimer’s disease, their function can be implicated in disease progression. The intricate nature of studying microglia within the human brain poses challenges, hindering a comprehensive understanding of their involvement in Alzheimer’s disease.
A team of researchers led by Prof. Bart De Strooper (UK-DRI@UCL and VIB-KU Leuven) and Prof. Renzo Mancuso (VIB-UAntwerp) delved into this complexity by creating a xenotransplantation model. This model involved introducing stem-cell-derived human microglia into the brains of mice to observe how these cells respond to the disease environment. Their study, detailed in Nature Neuroscience, aims to shed light on the intricate mechanisms underlying Alzheimer’s disease progression.
Alzheimer’s disease is a multifaceted and progressive neurodegenerative disorder affecting millions globally. With projections indicating a drastic surge in cases by 2050, there is an urgent call for innovative treatment approaches.
Microglia, as the brain’s resident immune cells, are pivotal in clearing debris and addressing inflammation within the brain. In Alzheimer’s disease, the role of microglia is pronounced, particularly in the formation and initial response to amyloid-β plaques—a defining feature of the disease. These plaques incite a foreign body response from microglia, leading to the neuroinflammation characteristic of Alzheimer’s disease.
The conventional method of studying microglia in postmortem human brain samples presents challenges due to genetic variations among individuals, post-death intervals, and the presence of other brain conditions. As a result, investigations into the behavior of microglia in these samples have yielded inconsistent results. Moreover, assessing the impact of medications on postmortem brains is unfeasible.
To address these limitations, Dr. Nicola Fattorelli and Dr. Anna Martinez Muriana, along with colleagues from various research institutions, developed a unique mouse model. This xenotransplantation model, genetically modified to mimic amyloid-β plaque accumulation in Alzheimer’s patients, allows for the transplantation of stem-cell-derived human microglia.
Past research utilizing a similar model showcased the demise of transplanted human neurons in Alzheimer’s disease. The current study utilized this platform to explore the response of human microglia towards amyloid plaques throughout the disease progression.
The researchers observed that human microglia exhibited a more intricate immune reaction to amyloid-β compared to their rodent counterparts. Additionally, the genetic transition from a normal to a reactive state in human microglia differed from that in mice.
The study underlines the importance of considering variances in human and mouse microglia responses when exploring Alzheimer’s disease for potential therapeutic interventions. The research also highlighted the influence of genetic predispositions on how human microglia react to the disease, emphasizing their role in the disease process.
Moreover, the findings hinted at a potential interaction between microglia and soluble forms of amyloid-β, suggesting a possible impact on disease progression in the early stages of Alzheimer’s. The implications of this interaction on neurons and other brain cells, and its relevance to treatment strategies, remain areas for further exploration.
In conclusion, this study marks a significant stride towards unraveling the intricacies of Alzheimer’s disease. The insights gained on the diverse responses of human microglia in Alzheimer’s disease could pave the way for more effective treatment modalities. The researchers affirm the efficacy of the xenograft model as a valuable tool for studying the genetic underpinnings of microglial responses in Alzheimer’s disease.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it